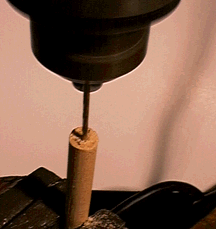
Cut off a 4 inch segment of the wooden dowel. Drill a small hole in one end of the segment.
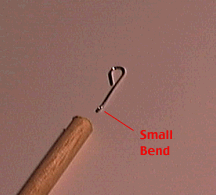
Bend a small (1 cm) loop in one end of the wire. Flatten this loop out with a pair of "needlenose" pliers so it makes a tight 180 degree bend. Make another, larger loop in the other end of the wire. Do not flatten this loop. Insert the small loop into the hole in the dowel. Test the wire to make sure it will not come loose from the dowel. If it is loose, bend the small loop out slightly and re-insert into the dowel.

Crush the rock sample into powder. Only a small amount is required.

Heat the platinum wire with the torch until it is red hot. Immediately place the wire into the sodium fluoride. The fluoride should adhere to the wire. Heat the powder until it has melted. Repeat this procedure, adding more fluoride until you have a small bead on the wire loop. While the bead is still molten, dip it into the rock powder. A few grains should adhere to the bead. Re-heat this and repeat one or two more times. Only a few grains of rock powder will color the bead quite well.

Allow the bead to cool. Turn off the lights in the room. This step must be done in darkness. Place the bead under the blacklight. If it fluoresces, chances are you have a uranium or thorium containing mineral or rock!