as most of the nitrogen remains chemically bound in the semi-coke, from which it can, however, subsequently be recovered in the form of ammonia by further heating or by gasification. The gas from low-temperature carbonisation is rich in hydrocarbons, especially methane, and reaches a calorific value of 9000 calories per cb.m. (1000 B.Th.U. per cub. ft.).
Table II 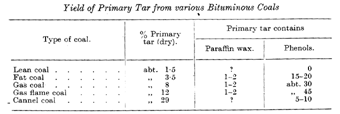
Table II shows the yields of low-temperature tars from different coals. The figures refer to low-temperature tar free of water, and to dry coal. Percentages of paraffln wax and phenol are also recorded.
It will be seen that bituminous coals proper, from lean coal to gas flame coal yield more primary tar the younger and hence the richer in oxygen they are, and that the primary tars contain more phenols the younger the coal from which they were prepared. The content of paraffin wax in the tar from bituminous coals ranges from 1 to 2 per cent. Cannel coal, essentially of sapropelic and not ulmic character, stands in a class bv itself. It is comparatively rare in Germany, but more frequent in England. High-grade cannel coals yield up to 30 per cent. of primary tar, which, as regards its paraffln and low phenol percentage, differs from the tar from bituminous coals and resembles somewhat that from lignites.
Table III 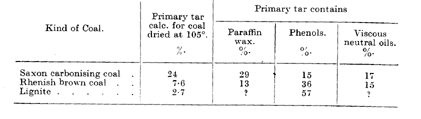
Table III relates to brown coals. Their higher paraffin wax and lower liquid phenol content is manifest from their pasty consistency at ordinary temperature. Unlike bituminous coal, brown coals give tars poorer in phenols and richer in paraffins as their total yield increases. This is clearly seen in Table III by comparing the Saxon carbonising coal with lignite.
In Table IV the yields of primary tars of different kinds of peat are recorded. The figures here vary as they do in the case of the lignites. The Lauchhammer peat, in particular, shows extraordinarily high tar yields.
Table IV 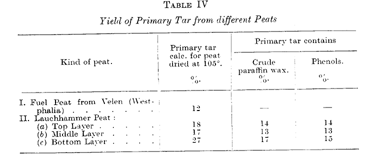
Fig. 7 gives the composition of the primarv tar of gas flame coal in diagrammatic form. It shows at a glance that approximately half the tar consists of constituents soluble in alkali, mainly higher phenols and acid resins. The other half (containing paraffin) consists of neutral compounds and only about 1 percent of bases.
On distillation at ordinary pressure, the constituents boiling above 300 degrees, i.e., more than half the tar, would undergo a far- reaching decomposition. It is therefore advisable to distill in vacuo or with superheated steam. The first fraction of about 10 per cent. is tar benzine boiling below 200 degrees. The phenols having been previously separated, there follow limpid oils, viscous neutral oils and
Figure 7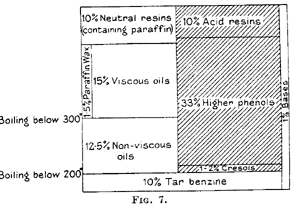
Go Back One Page

 Go Forward One Page
Go Forward One Page
Return To Main



